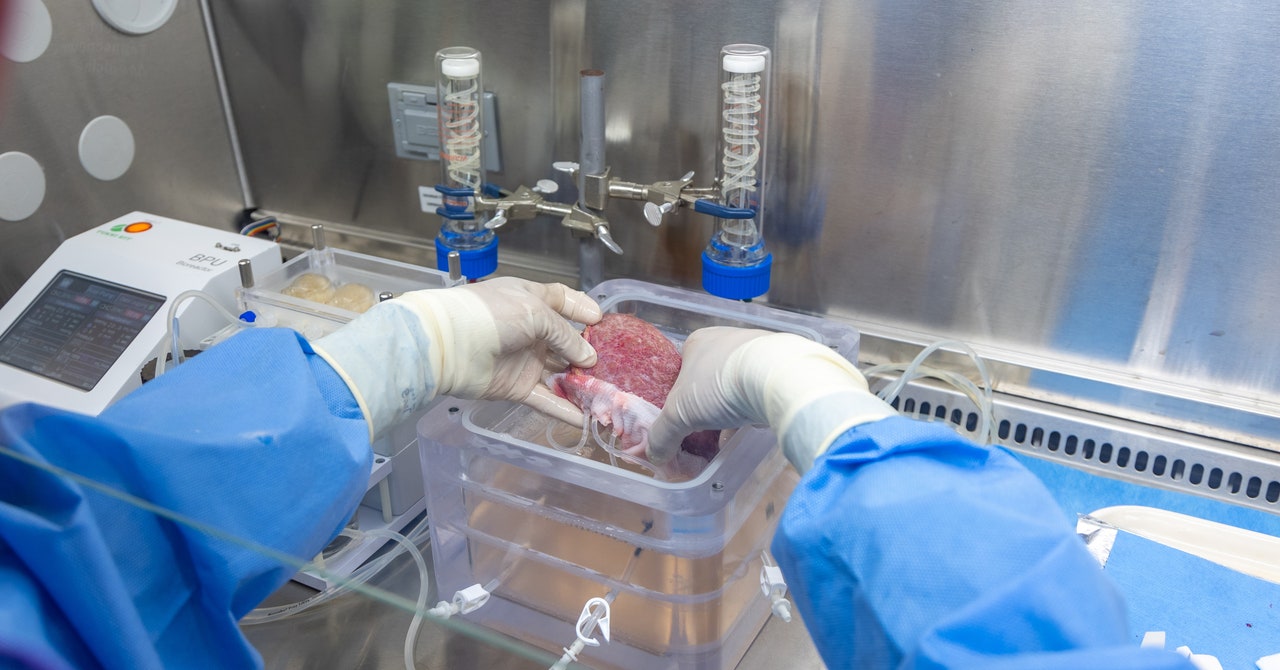
A healthy liver is spongy and reddish-brown in color with a smooth appearance. But when the surgeons took this one out of the cooler, it was hard, marbled, and covered in bumps—evidence of cirrhosis, a type of end-stage liver disease. Over time, the man’s healthy liver cells had been replaced by scar tissue, and eventually, his liver stopped working. His only option was to get a new one.
Livers are the second most in-demand organ. In 2023, a record 10,660 liver transplants were performed in the US, driven in part by a steadily growing number of living donors. In a living liver transplant, a piece is taken from a healthy person’s liver and transplanted into a recipient. But even with this uptick in transplants, not everyone who needs a new liver receives one. Patients may have other health problems that disqualify them from a transplant, and others may die while waiting for one. In 2022, the latest year for which data is available, the Centers for Disease Control and Prevention recorded nearly 55,000 deaths due to chronic liver disease.
Living donor transplants are possible because of the liver’s unique capacity to regenerate itself—more so than any other organ in the body. In a healthy person, the liver can regrow to its normal size even after up to 90 percent of it has been removed. But disease and lifestyle factors can cause permanent damage, rendering the liver unable to repair itself.
When Soto-Gutiérrez was studying medicine at the University of Guadalajara in Mexico, his uncle died of liver disease. From then on, he became dedicated to finding a treatment for patients like his uncle. In the early years of his medical career, he noticed that some patients with scarred livers were bound to a hospital bed waiting for a transplant, while other people with cirrhosis were walking around, seemingly living normal lives. He figured there must be cellular differences in these livers.
He teamed up with UPMC transplant surgeon Ira Fox to look for transcription factors—master regulators that can dial up or down the expression of groups of genes—that can potentially reprogram injured organs. Genes rely on transcription factors to perform many essential functions in organs. Together, Soto-Gutiérrez and Fox have analyzed more than 400 failing livers donated by transplant patients. When they compared them with dozens of normal donated livers that acted as controls, they identified eight transcription factors essential for organ development and function.
They zeroed in on one in particular, HNF4 alpha, that seems to act like a main control panel, regulating much of the gene expression in liver cells. In healthy liver cells, levels of HNF4 alpha were turned up, and so were other proteins it controls. But in the cirrhotic livers they examined, HNF4 alpha was almost nonexistent.
The team needed a way to get the transcription factor into liver cells, so they turned to mRNA technology. Used in some of the Covid-19 vaccines, mRNA is a molecule that carries instructions for making proteins, including transcription factors. In the Covid vaccines, the mRNA codes for a part of the virus known as the spike protein. When injected into a person’s arm, the mRNA enters cells and kicks off the protein-making process. The body recognizes these spike proteins as foreign and generates antibodies and other defenders against it.